Summer 2020 CRANE Superuser Training Opportunity
The UNMC Clinical Research Analytics Environment (CRANE) consists of the policies, procedures, and technology to allow secondary use of Nebraska Medicine EHR data for research. The system is an IRB approved data registry. By removing all patient identifiers the resulting data set does not require additional IRB approval for additional analysis. However, Nebraska Medicine and UNMC security and compliance policies require tight control over access and release of data.
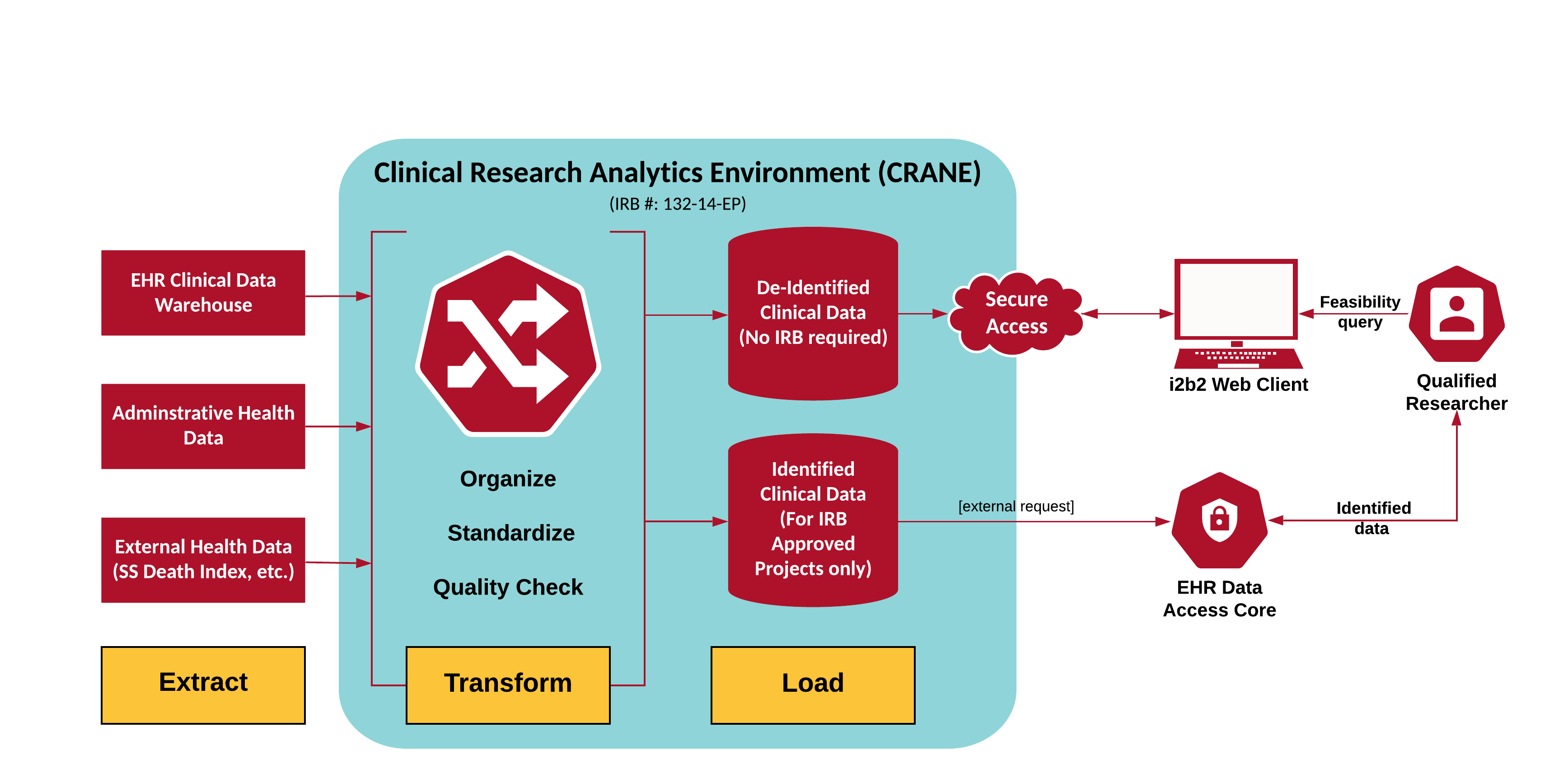
UNMC’s CRANE provides researchers with access to well organized, characterized and standardized patient-level data in compliance with HIPAA, the common rule, and best practices. The initial architecture for CRANE was developed by the Nebraska Medicine and UNMC Governance Enterprise Data Sharing (GEDS) Committee, led by Dr. Kratochvil, in 2011 during the acquisition of EPIC at Nebraska Medicine. The opportunity to build out the infrastructure arose when UNMC joined KUMC to form the Greater Plains Collaborative (GPC) Clinical Data Research Network with funding from. The technical platform was built based on the successful KUMC Heron i2b2-based infrastructure.
The core CRANE data derives from the Nebraska Medicine EHR. The CRANE team developed a suite of software tools to extract and transform the data into well-characterized common data models. The EHR data is supplemented with other patient-level data sources including, but not limited to, state cancer registry data and the SS death index.
Additional data sources to expand the patient-level data include social determinants of health, formally encoded anatomic pathology data, biomarker data, and pointers to biobank specimens.
Access to the system is limited to UNMC faculty or their designees who have completed the CITI Good Clinical Practice (GCP) course and are in compliance with UNMC competency training including HIPAA training and Information Security Awareness training. In addition, they must submit a signed agreement (see Appendix A) attesting to their understanding of the compliance rules of CRANE and their intended use of it. For sponsored users, their department or unit chair must sign authorizing them for access. These researchers may apply for access to the de-identified data system for feasibility queries or de-identified data requests. Access to identified patient data requires IRB approval. Data release is through an honest broker for qualified faculty.
There are two levels of CRANE access: web client and Superuser. Table 1 below shows the different requirements for obtaining the levels of access. Web client access is the primary mode through which most CRANE users will interact with the data. This allows for easy cohort selection using the hierarchy defined by the i2b2 workbench and feasibility studies.
Superuser access allows for direct access to the de-identified tables within CRANE using MS SQL Server and writing queries. These users have more in-depth training and a greater understanding of the underlying data structures in CRANE. They serve as departmental resources for users in their areas, train users in the basics for web client access, and can initiate data pulls for research questions by writing queries and submitting them through a pre-defined process for the CRANE Database Administrator to validate and pull after verifying the veracity of the query as it relates to the research question.
Web Client User | Superuser |
|
|
 Brendan Cope, Pharm.D, is a post-doctoral research associate in the Division of Rheumatology and a member of the CRANE Superuser community. He describes the impact of CRANE in the Great Plains as the following: “We members of the CRANE research team are proud of our database. Every day our database helps expand the influence of UNMC research. As the world continues to become smaller and ever-increasing amounts of data are stored in many formats, we stand ready to help answer the needs of our researchers with CRANE.”
Brendan Cope, Pharm.D, is a post-doctoral research associate in the Division of Rheumatology and a member of the CRANE Superuser community. He describes the impact of CRANE in the Great Plains as the following: “We members of the CRANE research team are proud of our database. Every day our database helps expand the influence of UNMC research. As the world continues to become smaller and ever-increasing amounts of data are stored in many formats, we stand ready to help answer the needs of our researchers with CRANE.”
If you would like to learn more about CRANE, becoming a member, or joining the Superuser community, please reach out to the Data Access/Program Coordinator Jerrod Anzalone, alfred.anzalone@unmc.edu.



